Brief
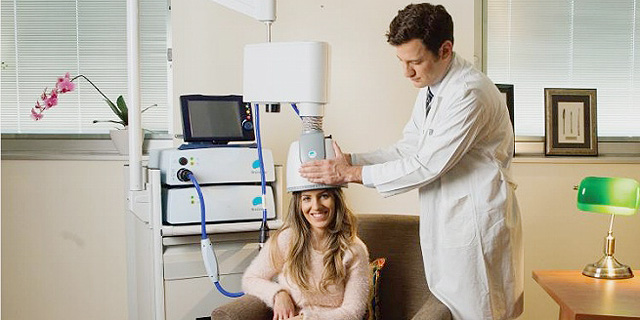
FDA Approves Brainsway’s Magnetic Stimulation Headset for OCD Treatment
Brainsway develops non-invasive medical devices that are worn on the head like helmets to apply magnetic stimulation to treat brain disorders
CTech | 16:24, 19.08.18
The U.S. Food and Drug Administration on Friday approved for marketing a magnetic stimulation headset by Jerusalem-based medical device company Brainsway Ltd. for the treatment of obsessive-compulsive disorder (OCD).
Brainsway develops headsets that use magnetic stimulation to treat brain disorders. The company's devices are already cleared by the FDA for the treatment of depression, and by the European Union for various disorders including stroke rehabilitation, PTSD, Alzheimer's disease, and smoking cessation.
OCD is a common and chronic disorder which causes a person to have uncontrollable, reoccurring thoughts and an urge to repeat certain behaviors over and over. It is typically treated with medication and psychotherapy. Transcranial magnetic stimulation, in which magnetic fields are used to stimulate nerve cells in the brain, is already FDA-approved as a treatment for depression and migraine headaches.
The Brainsway stock currently is up over 14% on the Tel Aviv Stock Exchange.
No Comments Add Comment