Bio-convergence
Small wonders: Israeli companies tackling unmet clinical needs, one implant at a time
Bio-convergence could be Israel’s next big growth engine, offering solutions from artificial retina implants that restore vision to an electronic patch that detects neural disease before symptoms appear
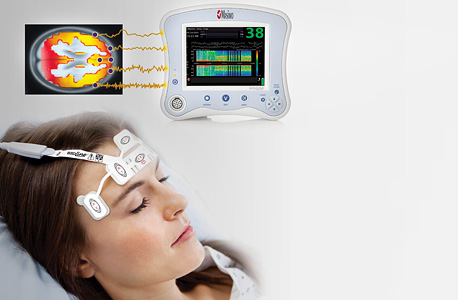
"Sleep disorders are one of the accurate early indicators of neurological diseases and we believe that our product can help in the early identification of Parkinson's disease, even several years before motor symptoms appear," Hanein said.
Typically, the rapid eye movement (REM) sleep of healthy individuals comes with reduced muscle activity, while that of most Parkinson's patients is characterized by increased activity. This phenomenon is called REM sleep behavior disorder (RBD) and the only way to monitor it is by examining neural and muscle activity during sleep. "This phenomenon is episodic and does not occur every night," Hanein explained, "so, it is very important to perform the measurements continuously for several nights and at optimal conditions. Using the sticker, patients can be monitored at home and the data is received by the medical team supervising the test via the mobile app. The common alternative is a sleep laboratory, which is a costly process that requires constant medical personnel supervision.” According to Hanein, developing the technology is just the beginning of the company’s journey. “We still need to conduct a clinical trial that proves that the product works,” she said. “This requires substantial capital and we are currently collaborating productively on this with Anat Mirelman at the Tel Aviv Sourasky Medical Center (Ichilov Hospital). For now, an experienced operator must visit the patients' home and help them connect to the system but we are also working simultaneously on converting the system to be self-operated." According to Hanein, electrophysiological monitoring is customarily used in many areas of medicine but the process, when conducted in a lab or a hospital, involves inconvenient electrodes that have many disadvantages. The electrodes tend to fall off, they can only operate for a limited time, and the data received is not always accurate. On the other hand, Hanein said, X-trodes’ technology does not cause discomfort to patients and can be used for hours at a time, even during the night. The next stage: processing emotions and facial expressions"We are still working on integrating additional sensors into the system," Hanein said, "but at present, the electrode can successfully record muscle, eye, heart, neural, and diaphragm cells. We are still looking into ways to adapt the length of time the sticker is worn to ongoing or intensive activity." This endeavor has received financial support from IIA.
According to Hanein, the development of the company's products was made possible thanks to three fields that have evolved dramatically in recent years, the combination of which created a real bio-convergence opportunity—printed electronics, the miniaturization of specially designated electronics for electrophysiological signals, and AI.The smart sticker technology developed by X-trodes can be used in different areas, including psychiatry, psychology, behavioral science, business management, motor disorders, stuttering, and autism, Hanein said. "We understood that there was great interest in trying out our technology and when we founded the company we decided to build and develop a research system in addition to developing the company's primary products," she added.
One of the interesting areas still being studied in the research labs that Hanein heads is the processing of facial expressions and monitoring of emotions. As she explained, the initiative focuses on identifying objective indices for defining emotions and their intensity among different people. "There are physiological indices such as perspiration and pulse acceleration, but these are not definitive,” she said. “Using different sensors, we try to measure facial expressions and to combine the data with additional physiological indices to make it useful."Bio-Convergence—A Growth Engine for Israel
Hanein also serves as vice president of scientific affairs at Israel-based Nano Retina Inc., which develops an artificial retina implant that mimics the natural physiological processes of the human eye and restores functional vision to people blinded by retinal degenerative diseases.
Nano Retina is part of Rainbow Medical Ltd, an innovation and investment company that develops and invests in medical technologies addressing the most significant unmet clinical needs, including Alzheimer's, blindness, diabetes, heart failure, hypertension, and pain. "Rainbow Medical's diverse portfolio of medical device companies are the epitome of the bio-convergence concept, fully utilizing the different fields of expertise and strengths in Israel's workforce," Director of Operations Yuval Mandelbaum said. "Once a new solution is vetted by physicians and has a solid proof-of-concept, a subsidiary company is incorporated by Rainbow Medical, and a diversified and experienced team is recruited to transform the idea into a commercial product,” Mandelbaum explained his company’s mode of operation. “To allow portfolio companies to focus on product development and clinical studies and reach the market as quickly as possible, Rainbow Medical offers financial support as well as expert hands-on guidance and centralized services, on-going market validation, and access to a vast network of key opinion leaders (KOLs) and strategic partners worldwide,” he said. According to Mandelbaum, this unique model was designed to allow for both capital and time efficiency, cutting costs, and expediting processes. The company's internal ideation stems primarily from the inventions and innovations of Yossi Gross, a luminary medical device entrepreneur with multiple success stories under his belt as well as more than 750 patents to his name. "Bio-convergence is part of Rainbow Medical's DNA, and we believe that when you have a truly diversified team you gain access to a higher level of innovation and the whole business becomes much greater than the sum of its parts,” Mandelbaum said. As such, Rainbow Medical's personnel is made up of multidisciplinary professionals from different walks of life, including biologists, engineers, physicists, and programmers all working in unison with experts in fields of nanotechnology, materials science, genetic engineering, and neurobiology, he said. Inspired by similar notions, IIA defines bio-convergence as one of Israel's next growth engines. “The strengths of Israel's industry are in areas like engineering, big data, and AI, alongside the fields of science that have flourished over the past 20 years and the rise of medical device and digital health that has characterized the last decade. Combine all these together and we will truly have a growth engine that will drive the economy for years," Mandelbaum said.Artificial retinas restoring sight to the blind
Nano Retina’s implant is equipped with an imaging chip that receives external visual input from the environment and converts it to electrical signals that are delivered to healthy layers through a dense array of hundreds of micro-electrodes that stimulate the bi-polar layer of the retina. The stimulation of the bi-polar cells allows the device to bypass the degenerated layer of photoreceptor cells and for visual signals from the outside environment to once again be detected as electrical signals by the retina and optic nerve, thus restoring functionality to the visual system.
At this stage, Nano Retina is focused on two conditions, retinitis pigmentosa and age-related macular degeneration (AMD)," Mandelbaum said. "These diseases cause people in mid-life to gradually lose their sight,” he said, adding that over 170 million people in the world currently suffer from these conditions. Nano Retina's device has entered the clinical phase and the first patients received their implants earlier this year, Mandelbaum said. “Early results are remarkable, as patients who were completely in the dark prior to the implantation now experience visual perception and are able to identify objects around them and carry out different day-to-day life activities unaided,” he said. Visual perception is expected to continue to improve over the coming months, Mandelbaum said, but this does not mean that patients' vision will return to 100% of what it once was. However, going from total darkness to being able to identify a doorway or recognize the face of a person talking to you and regaining independence is an exciting achievement with incredible benefit to patients, he said.Over the last few years, Nano Retina has been granted several research grants from IIA.
A rainbow of achievementsBelow are some of Rainbow Medical’s other bioelectronics and neuromodulation portfolio companies.
BlueWindBlueWind Medical Ltd. develops a neuromodulation platform, based on a miniature wireless implant for the treatment of multiple neurological disorders. The device was granted the European Union’s CE Mark for the treatment of overactive bladder (OAB) and chronic neuropathic pain. The company is currently performing a pivotal trial to secure regulatory approval in the U.S.
Vascular Dynamics
Vascular Dynamics Inc. develops a miniature, minimally invasive implant treating resistant hypertension by amplifying the natural baroreceptor response in the carotid sinus. The device has been implanted in more than 80 patients thus far and has shown consistent reductions of systolic values by over 20 mmHg on average, maintained for years.
EnopaceEnopace Biomedical Ltd. develops an endovascular neuromodulation device for the treatment of heart failure. The minimally invasive transcatheter device is placed in the descending aorta to improve the efficiency of the heart’s function by restoring autonomic balance and ventricular-arterial compliance. The company is performing a clinical study in several locations in Europe and Israel.
ChroniSenseChroniSense Medical Ltd. develops a medical-grade wearable device for chronic disease management and remote monitoring of patients’ vital signs, including blood pressure, oxygen saturation, temperature, respiratory rate, and electrocardiography. The device allows for hospital-grade patient monitoring from afar and enables the early detection of life-threatening conditions.
GluSenseGluSense Ltd. develops a fully implantable long-term continuous glucose monitor (CGM), with biological sensors, designed to allow Type 1 and Type 2 diabetes patients to accurately monitor glucose levels without the hassles associated with an external device.
BrainBalanceBrainBalance develops a minimally invasive device for the treatment of Alzheimer’s disease. The device creates a directional electric field that enhances the natural clearance process of amyloid-beta proteins (Aβ) out of the brain and into cerebrospinal fluid (CSF). The unregulated buildup of Aβ in the brain is associated with the symptoms of Alzheimer’s.
DisCureDisCure develops a miniature implant for the treatment of disc degeneration disease, one of the most common causes of lower back pain. The implant actively regulates the inflow of fluid and nutrients into the disc, restoring the cushioning function, improving inner disc-cell conditions, and reducing lactic acid and associated pain. DisCure is the only treatment designed to not only reduce pain but reverse the degenerative process of the disease.
The article was written in collaboration with the Israel Innovation Authority, responsible for the country’s innovation policy. Its role is to nurture and develop Israeli innovation resources, while creating and strengthening the infrastructure and framework needed to support the entire knowledge industry.