Tel Aviv University researchers 3D-bioprint an entire active tumor
Researchers were able to print a 3D model of a glioblastoma, the deadliest type of brain cancer, from human tissues. Breakthrough will enable faster tumor diagnoses, help provide swifter treatment for patients, and more accurate drug developments
The study was led by Prof. Ronit Satchi-Fainaro from the Sackler Faculty of Medicine and Sagol School of Neuroscience, who serves as the director of the Cancer Biology Research Center, is Head of the Cancer Research and Nanomedicine Laboratory, and director of the Morris Kahn 3D-BioPrinting for Cancer Research Initiative at Tel Aviv University. The new technology was developed by PhD student Lena Neufeld, alongside researchers at the team’s lab, including Eilam Yeini, Noa Reisman, Yael Shtilerman, Dr. Dikla Ben-Shushan, Sabina Pozzi, Dr. Galia Tiram, Dr. Anat Eldar-Boock, and Dr. Shiran Farber.
The 3D-bioprinted models are based on samples taken directly from patients from operating rooms at the neurosurgery department at Sourasky Medical Center.
Glioblastoma is a particularly aggressive disease, partially because it is unpredictable, Satchi-Fainaro explained. Furthemore, such tumors can remain dormant in some patients for years. The team was able to grow the tumors artificially in a lab, where they discovered that they all grew and spread at the same rate. However, in the 3D-bioprinted tumor’s spread and development was similar to what doctors see in patients or animals.
"Glioblastoma accounts for most brain malignancies," noted Prof. Satchi-Fainaro. "In a previous study, we identified a protein called P-Selectin, which is produced when glioblastoma cancer cells encounter microglia – or cells of the brain's immune system. We found that this protein is responsible for a failure in the microglia, causing them to support rather than attack the deadly cancer cells, helping the cancer spread. However, we identified the protein in tumors removed during surgery, but not in glioblastoma cells grown on 2D plastic petri dishes in our laboratory. The reason is that cancer, like all tissues, behaves very differently on a plastic surface than it does in the human body. Approximately 90% of all experimental drugs fail at the clinical stages because the success achieved in the lab is not reproduced in patients."
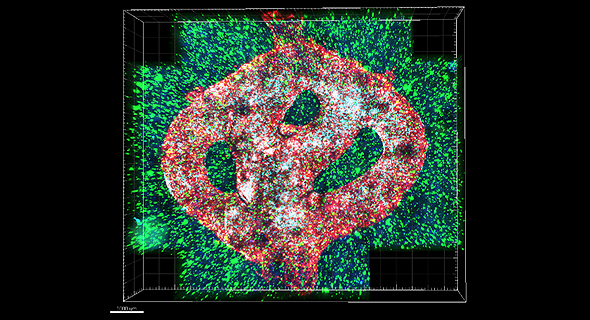
To address this problem, the research team created the first 3D-bioprinted model of a glioblastoma tumor, which includes 3D cancer tissue surrounded by an extracellular matrix, which communicates with its microenvironment and functional blood vessels.
"It's not only the cancer cells, but all cells in the brain’s microenvironment; the astrocytes, microglia and blood vessels are connected to a microfluidic system – which enables our brains to deliver substances like blood cells and drugs to the tumor replica. Each model was printed in a bioreactor we have designed in the lab, and uses a hydrogel sampled and reproduced from the extracellular matrix taken from the patient, thereby simulating the actual tissue.”
After successfully printing the 3D tumor, the team demonstrated that unlike cancer cells growing on petri dishes, the 3D-bioprinted model has the potential to be more effective for rapid, robust, prediction of a treatment that could be personalized per patient.
"If we take a sample from a patient's tissue, together with its extracellular matrix, we can 3D-bioprint from this sample 100 tiny tumors and test many different drugs in various combinations to discover the optimal treatment for this specific tumor. Alternatively, we can test numerous compounds on a 3D-bioprinted tumor, and decide which is most promising for further development and investment as a potential drug. But perhaps the most exciting aspect is finding novel druggable target proteins and genes in cancer cells, which is a very difficult task when the tumor is inside the brain of a human patient or model animal. Our innovation gives us unprecedented access, with no time limits, to 3D tumors mimicking the actual tumor, enabling optimal investigation,” she added.
The study was funded by the Morris Kahn Foundation, the European Research Council, the Israel Cancer Research Fund, the Israel Cancer Association, the Israel Science Foundation, and Check Point Software Technologies Ltd.